✔ 100% Authentic Product
👁️ Currently Viewing 10738
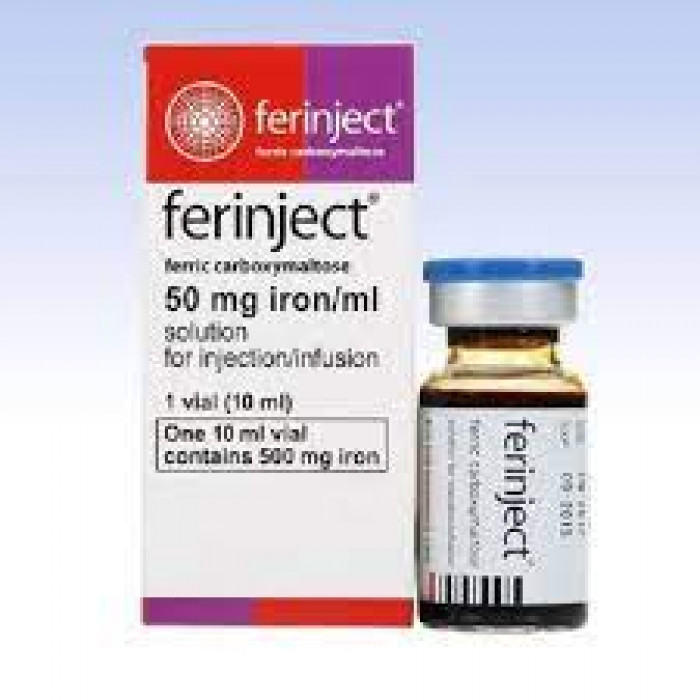
Ferinject IV Injection or Infusion 500mg/10ml
Treats iron deficiency anemia when oral iron is ineffective or unsuitable.
📄Prescription Required
Discount
Price: ৳ 3,327
MRP:
৳
3430
3%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
Ferinject 500 contains Ferric carboxymaltose, a medication classified under Iron replacement medicines. It is prescribed to treat iron deficiency anemia (IDA) in adults and adolescents above 14 years old who cannot tolerate oral iron supplements, require rapid iron supplementation, or have non-dialysis-dependent chronic kidney disease. Iron deficiency anemia occurs when the body lacks sufficient iron, necessary for the production of red blood cells and oxygen transportation.
Before initiating treatment with Ferinject 500, your doctor may recommend relevant tests to assess your condition. Inform your doctor if you are pregnant, planning to become pregnant, or breastfeeding. Ferinject 500 is not suitable for children and adolescents under 14 years old, and caution should be exercised when administering it to elderly patients aged 65 years or above. Seek advice from your doctor before starting Ferinject 500.
Safety Advices

Alcohol
UNSAFE
It's uncertain if it's safe to consume alcohol with Ferinject 500. Please consult your doctor.

Pregnancy
CONSULT YOUR DOCTOR
Ferinject 500 may be unsafe during pregnancy, as limited human studies suggest potential harm to the developing baby. Consult your doctor to weigh the benefits and risks.

Breastfeeding
CONSULT YOUR DOCTOR
Ferinject 500 is safe during breastfeeding. Studies show minimal transfer to breastmilk, posing no harm to the baby.

Driving
CAUTION
Ferinject 500 usually doesn't affect your ability to drive.

Kidney
CONSULT YOUR DOCTOR
Likely safe in patients with kidney disease, as dose adjustment may not be necessary. Consult your doctor.

Liver
CAUTION
Use with caution in patients with liver disease, as dose adjustment may be needed. Consult your doctor.
✔️ Uses of Ferinject IV Injection or Infusion 500mg/10ml
Treatment of iron deficiency anemia.
✔️ How does Ferinject IV Injection or Infusion 500mg/10ml work?
Replenishes iron stores in the body, vital for forming new red blood cells and hemoglobin.
✔️ Side Effects of Ferinject IV Injection or Infusion 500mg/10ml
Common side effects include nausea, hypertension, flushing, decreased blood phosphorus, dizziness, vomiting, pruritus, rash, urticaria, wheezing, injection site discoloration, headache, increased alanine aminotransferase, dysgeusia, hypotension, constipation, and serious anaphylactic/anaphylactoid reactions.
✔️ Quick Suggestions:
- Ferinject 500 is used when oral iron fails.
- Injection may cause allergic reactions; observe for 30 minutes after each dose.
- Regularly monitor blood pressure and iron levels.
- Inform your doctor of any oral iron supplements.
- Avoid alcohol consumption during treatment.
- Stool discoloration is normal.
- Hypersensitivity and hypertension may occur; monitor accordingly.
✔️ Indication of Ferinject IV Injection or Infusion 500mg/10ml
Ferric Carboxymaltose is prescribed for treating iron deficiency anemia in adult patients who:
- Cannot tolerate oral iron or have had an inadequate response to it.
- Have non-dialysis dependent chronic kidney disease.
✔️ Tips:
- For patients weighing 50 kg or more: Administer Ferric Carboxymaltose in two doses spaced at least 7 days apart, with each dose being 750 mg, not exceeding a total cumulative dose of 1500 mg of iron per course.
- For patients weighing less than 50 kg: Administer Ferric Carboxymaltose in two doses spaced at least 7 days apart, with each dose being 15 mg/kg body weight, not exceeding a total cumulative dose of 1500 mg of iron per course.
- Ferric carboxymaltose is given intravenously either as a slow push or by infusion. For infusion, dilute up to 750 mg of iron in 250 mL of sterile 0.9% sodium chloride injection, ensuring a concentration of at least 2 mg of iron per mL, and administer over 15 minutes.
- The solution is stable for 72 hours when stored at room temperature after being added to an infusion bag.
✔️ Dosage of Ferinject IV Injection or Infusion 500mg/10ml
Dose: Adults: 750 mg IV once, followed by a second dose 7 days later; dosage adjustment for weight may be necessary.
✔️ Administration of Ferinject IV Injection or Infusion 500mg/10ml
Given intravenously (IV) by infusion or injection.
✔️ Interaction
No formal drug interaction studies have been conducted with Ferinject.
✔️ Contraindications
Hypersensitivity to any component.
✔️ Pregnancy & Lactation
- Ferric Carboxymaltose falls under Pregnancy Category C, with animal studies showing adverse effects on the fetus. Use in pregnant women may be considered if the potential benefits outweigh the risks.
- Caution is advised during breastfeeding due to limited data on its safety.
✔️ Precautions & Warnings
- Monitor patients for signs of hypersensitivity, including anaphylactic reactions, during and after administration.
- Watch for transient elevations in systolic blood pressure, which may occur immediately after dosing.
- Ferric Carboxymaltose belongs to the therapeutic class of Parenteral Iron Preparations.
✔️ Storage Conditions:
Store Ferric Carboxymaltose below 30°C and avoid freezing.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.